Intermediate Clinical Research Fellowships
Entry requirements
- Successful PhD (or MD) plus NTN and at least two years completed specialist training, but usually no more than two years after CCT.
- NTN will be exchanged for NTN(A) on appointment, and the individual may progress to consultant level during the Fellowship.
- Get to know some of the BHF-funded researchers here to learn more about their lives, inspirations and research interests.
- Residency requirements do not apply.
- Intermediate Clinical Research Fellows are eligible to apply for accelerated endorsement of the UK Global Talent Visa. More information on the UK Global Talent Visa
Grant overview
- Five years duration, with the possibility of a two year extension. More information on how to apply for an extension to a Fellowship.
- Part of the award may be spent overseas subject to a justified case being made.
- In the established UK-based home institution, the candidate must have the support of a named senior investigator who will guarantee access to space and resources and provide scientific guidance for the duration of their Fellowship. This individual should be named on the application form as a 'Supervisor' and provide an appropriate letter of support as part of the application.
- In an overseas/second UK institution, the candidate must additionally have the support of a named senior investigator who will guarantee access to space and resources and provide scientific guidance for the duration of the stay. This individual should provide an appropriate letter of support as part of the application.
- The Fellowship may be taken up on a part-time employment basis, where appropriate, following discussion and agreement with us and the employing institution. For more information, read about our Flexible working policies.
Award may include
- Salary of applicant (if after CCT, up to 10 PAs).
- Fellows should include justification of clinical sessions with their submitted job plan. The BHF will normally allow up to 2 sessions (2 PAs) to be spent on clinical activity not directly contributing to the proposed research project. The BHF recognise that more clinical sessions may be appropriate to deliver the research, including when the project is highly patient-orientated in nature. Such requests will be considered on a case-by-case basis and will require a clear justification.
- Salary for technician or research assistant if required and fully justified.
- Research consumables, directly attributable to the project. Consumables will not normally be provided overseas; facilities should be provided by the host department.
- Research equipment essential for the project. Equipment will not be provided overseas; facilities must be provided by the host department.
- Travel funding of up to £500 per year to present research at or attend scientific meetings relevant to the grant.
For overseas visits of up to six months:
- Economy class travel expenses for the Fellow only.
- A justified subsistence allowance.
For overseas visits of more than six months:
- One return economy fare for the Fellow and immediate family.
- A reasonable contribution to healthcare insurance (e.g. applicable to USA).
- Housing contribution up to £3,000.
How to apply
- Read the information in How to apply.
- Read our commitment to Improving Support for Clinical Academics.
- If you are applying for funding for a clinical study, also read:
- Log onto the online application form.
- Complete all the sections of the online application form.
- The online application must be completed by the applicant.
- Short-listed candidates may be interviewed.
Case for Support
You will be asked to attach a single PDF document to your online application form containing the following information:
- For resubmissions, include an unedited copy of the original feedback followed by a detailed response, limited to 3 sides of A4, explaining how the revised application has changed from the original submission.
- Title of the proposed research.
- Abstract of the proposed investigation in 200 words or less.
- Background to the project and pilot data.
- Original hypothesis.
- Experimental details and design of proposed investigation.
- If requesting funding for a clinical study, include all information listed in the Clinical Study Guidelines (Interventional Study) or in the Clinical Study Guidelines (Observational Study). If you do not provide the information outlined in the guidelines, your application may be returned without formal consideration.
- Power calculations.
- Expected value of results.
- List of references relevant to the proposed project.
- Appendices should be a maximum of 3 sides of A4 containing only tables or figures essential to the understanding of the application. Research papers whether published, in press or in preparation may not be attached (although hyperlinks can be provided).
- Post-CCT applicants: a timetable of research and clinical activity.
Sections 2-9 must not exceed six A4 sides. The PDF file size must not exceed 20MB.
Please ensure Arial font size 12 is used. If this font size is not used, the application will be rejected prior to formal consideration.
Additional document uploads required on Case for Support page of the application form:
- Full CV of the candidate including list of publications and academic email address.
- Letter(s) of support from senior investigator(s) named as Fellowship supervisor(s).
- Letter of support from Head of Department where the Fellowship will be based, showing the academic institution's commitment to the applicant, including confirmation of any financial and career development support they will be providing.
- If a second institution or overseas visit is proposed, include a letter of support from a senior investigator who will provide access to space, resources and scientific guidance for the duration of the stay. Please also ensure that the purpose of the visit is explained clearly, in terms of the duration, the research to be carried out and any training that will be acquired.
- Pre-CCT applicants: a statement from the Postgraduate Dean, clearly explaining the type and amount of clinical training to be carried out during the fellowship.
Additional document uploads that will be requested within pages on the application form:
- Supporting letters from collaborators.
- Intellectual property agreements where relevant.
- Valid copy of ethical approval if relevant.
- Brief CV (two A4 sides) of named staff for whom salary is requested.
- Quotes obtained for any requested equipment with a value of £5,000 or more.
The BHF is prepared to consider funding or part-funding Intermediate Clinical Research Fellowships that include research experience with an industry partner (for example, experience of experimental medicine). Please contact our office for advice if you are considering applying for a co-funded Intermediate Clinical Research Fellowship.
Decision process
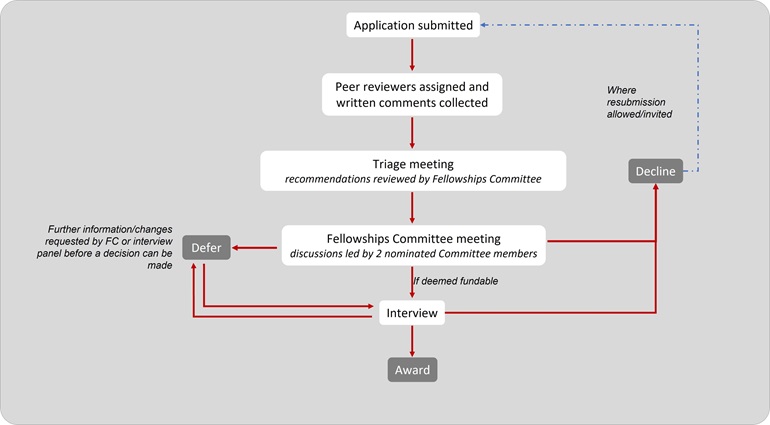
There are no closing dates. Please submit the application when it is ready and allow up to 6 months from submission to decision.
Receipt will be acknowledged within 24 hours and we will advise you when you can expect the result. Candidates may be interviewed and a decision on this will be reached by the Fellowships Committee (four meetings a year) after it has considered external peer review reports.
The flowchart below shows the application journey
Independent expert review
To help applicants better understand how their proposals are assessed, please see BHF independent review guidelines and blank review form.
Apply for research grants
