Patient and Public Involvement in Research
The importance of Patient and Public Involvement (PPI) is widely recognised within health and social care research. It is increasingly an expectation, if not a requirement of research funders, including British Heart Foundation (BHF).
The BHF Strategy to 2030 demonstrates our strong commitment to involving people with a lived experience of heart and circulatory conditions and their risk factors across all areas of our work. Additionally, we want to support and encourage the research community to conduct meaningful PPI by working in partnership with those affected by these conditions at all stages of the research cycle.
What’s on this page
Use the links below to jump straight to the information you require:
- What is Patient & Public Involvement in research, and what are the benefits?
- How to involve patients and the public in your research
- Patient & Public Involvement in the Research Cycle
- Information for BHF grant applicants
- Tips for writing your Plain Language Summary
- How can BHF help?
- External resources
What is Patient & Public Involvement in Research?
PPI research refers to an active partnership between researchers, patients, carers and members of the public throughout the research process. The National Institute for Health and Care Research (NIHR) defines public involvement in research as research being carried out ‘with’ or ‘by’ members of the public rather than ‘to’, ‘about’ or ‘for’ them.
What are the benefits of Patient & Public Involvement in research?
By actively involving patients, carers, their families, and the wider public in research, we enrich our understanding and ensure that research outcomes directly benefit those affected by heart and circulatory conditions and their risk factors. This approach is an integral part of responsible, ethical and impactful research, aligning with the needs of those it aims to help.
The insights, experiences and perspectives of those affected by heart and circulatory conditions can help to (including but not limited to):
- Support the identification of new research topics and priorities
- Improve study design and delivery
- Improve participant recruitment, retention and protocol adherence
- Ensure more relevant, patient-centric study outcomes
- Facilitate the dissemination of research findings
- Challenge researchers’ aims and assumptions
- Increase individual/team motivation and renew connection to the cause
- Increase the transparency and accountability of the research process
- Improve funding success
How to involve patients and the public in your research
Step 1: Start early!
Early involvement ensures that your research addresses the needs of those it aims to benefit, making outcomes more relevant and impactful.
People with a lived experience of heart and circulatory conditions and their risk factors can provide valuable insights that help refine research questions, methodologies, protocols and patient-facing information, leading to more robust and effective study designs.
By only involving people towards the end of your project, PPI can often become a tick-box exercise, revealing issues that could have been addressed much earlier in the research process.
Step 2: Develop a PPI plan
Define the purpose of involvement:
Define the purpose of involvement and establish objectives: why you are involving patients, carers and/or the public and what you hope to achieve.
Clearly outline how and when (i.e. at what stage/s of the research process) patients, carers, and/or the public will be involved in your project. To ensure that PPI contributors make an informed decision about their involvement and understand what is expected of them throughout the project, ensure that the following are cleat to them at the outset:
- Purpose of the Project
- Roles and Responsibilities
- Project timelines and time commitment
- Payment and expenses policy (see Step 3: budgeting for PPI)
Identify who to involve:
- Determine who you want to involve and why they are the appropriate for your project. You should aim to involve people with a lived experience of the condition(s) and/or risk factors that you are researching. Depending on the project this could include patients, carers and/or members of the public.
- Consider how you will capture a diverse range of perspectives and experiences, including but not limited to; age, gender, ethnicity and geographic location (e.g. urban/rural/regional). This can increase the inclusivity of your research, representation of the broader population and reflect differing experiences of the condition(s) and access to care. Refer to relevant resources such as the Centre for Ethnic Health Research for guidance on Increasing diversity in research.
Refer to the BHF report on How inequalities contribute to heart and circulatory diseases for more information, and learn more about what steps we have taken to encourage inclusive research design by visiting Igniting Change: towards inclusive research design. - Once you have identified who you want to involve, it is important to plan your recruitment strategy:
- Advertise through established PPI networks such as BHF Heart Voices and other relevant charities (see How BHF can help)
- Advertise through platforms such as NIHR People in Research
- Engage local community groups, charities, GP surgeries, health services, NHS Trusts and/or patient support groups
- Determine whether your research institution has existing links to PPI groups and/or offers support with recruitment.
- Dependent on the role, you may wish to take an informal approach to recruitment e.g., “please share a bit about you, and why you are interested in the role” or a more formal approach e.g., application and/or interview.
Identify how to involve them
Choose from various involvement methods to gather patient and public insights, for example:
- Surveys
- Focus Groups
- Interviews
- Workshops
While surveys can be a good starting point, meaningful PPI is more than just collecting data and requires active engagement and collaboration throughout the research process. Focus groups, interviews, and workshops allow for interactive and iterative discussions, ensuring a deeper understanding of perspectives. A combination of these methods, where appropriate, can ensure a comprehensive involvement process that captures both the breadth and depth of patient and public perspectives.
The frequency of involvement will be dependent on project needs; however involvement methods can be used either as one-off activities or as part of a more sustained involvement process. These methods can be conducted face-to-face or online (e.g., via Teams or Zoom). For example:
- One-off Activities: Methods like surveys, focus groups, or interviews can be used to gather specific insights at a particular point in the project.
- Multiple Workshops: For projects requiring iterative feedback, multiple focus groups or workshops can be organised. These allow for ongoing discussions and deeper exploration of themes over time.
- Long-term Panels: Establishing long-term panels or advisory groups can provide continuous input throughout the project’s duration. This approach is beneficial for projects needing sustained engagement and evolving feedback.
Regularly assess the effectiveness of your chosen methods and be open to adjusting your approach in response to feedback from PPI Contributors and/or evolving project needs.
Step 3: Budget for PPI
- Ensure you allocate a sufficient budget for PPI activities. This should cover both out-of-pocket expenses (e.g., travel and accommodation for in-person meetings) and payment for participants’ time and expertise (honorarium). Expenses should be covered in advance where possible i.e. booking travel and/or accommodation on their behalf.
- Prior to involvement - ensure you have a clear understanding of what expenses and payments you can offer, how claims can be submitted (e.g., expenses form) and how payments will be made (e.g., via BACs). Ensure relevant finance processes are set up in advance to guarantee timely payments, ideally within 30 days. This information should be clearly communicated in your role profile and/or advertisement, so PPI contributors can make an informed decision about whether to get involved.
- Refer to our Grant Costing Guide for general guidance on the types of cost that may be requested on BHF grant applications. This includes costs relating to PPI e.g., payments for time (honorarium) and out-of-pocket expenses incurred when attending in-person meetings. For pre-application stage PPI, we recommend seeking advice from your research institutes relevant PPI or engagement staff.
Refer to NIHR payment guidance for researchers and professionals.
Step 4: Provide training and ongoing support
- Offer an induction/training session where appropriate, to help PPI contributors to understand the research process, the context of your project and their role in it. This is an opportunity to build relationships and create a welcoming and inclusive environment where PPI contributors feel valued and heard.
- Where required, seek PPI training for members of the research team e.g., find out if your research institution offers training and/or additional PPI support.
- Ensure PPI contributors know who they can contact if they have any additional queries and/or support needs throughout the course of the project and respond in a timely manner.
- Consider individual accessibility requirements to ensure that meetings and materials are accessible to everyone.
Step 5: Ensure effective communication and transparency
- Open and honest communication is key to building trust between researchers and PPI contributors. Transparency about the research objectives, processes, outcomes and how their feedback is being used throughout the project, helps create a collaborative environment based on mutual respect.
- When patients and the public feel informed, listened to and valued, they are more likely to actively participate and remain engaged with the research process.
Step 6: Feedback and evaluation
- Ensure there are feedback mechanisms in place (e.g., feedback forms), so that you can be responsive to queries and suggestions throughout the project. Regularly assess the involvement process and be willing to make improvements based on this feedback.
- Closing the feedback loop is essential for meaningful involvement and involves not only collecting feedback but also acting on it and communicating the outcomes back to the PPI contributors. Keep those involved updated about the progress of your research and how their contributions have made a difference.
- The level of impact and changes made as a result of PPI will differ depending on the project. However, even if some changes are not implemented, it is important to communicate and explain this to those involved.
Step 7: Recognise and reward contributions
- Remember to thank PPI contributors for their contributions throughout the project and celebrate successes.
- Acknowledge PPI contributions in internal and external reports, publications and presentations.
Step 8: Dissemination
- It is important to ensure that those who contributed to your research are informed about the outcomes, and how their input has made a difference. This acknowledges their time, efforts and contributions throughout the project.
- PPI contributors can also support the dissemination of research findings to wider audiences and ensure that information is presented in a way that is accessible and understandable for patients, carers and the public.
- When PPI contributors are involved in dissemination, it demonstrates a commitment to transparency and inclusivity. This can build trust and credibility with the wider community, as it shows that the research has been guided by those it aims to benefit.
Patient & Public Involvement in the Research Cycle
The diagram below demonstrates how patient and public involvement (PPI) can enhance your research throughout the lifecycle of a research grant - from identifying the research topic, to designing, developing and undertaking the research, to helping with the dissemination and implementation of research findings.
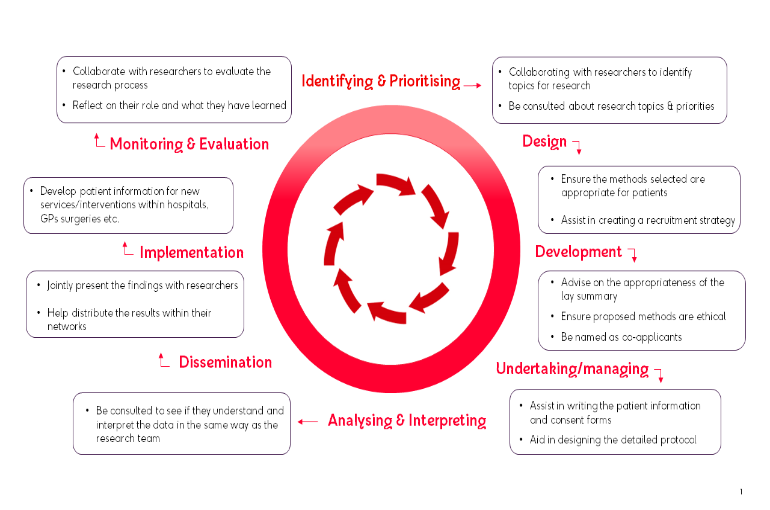
Information for BHF grant applicants
PPI is an essential requirement for the following BHF funding schemes (although we encourage PPI across all grants, where relevant):
We expect applicants to actively involve patients, carers and the public, in the design and development of clinical studies and implementation projects. We recognise that the nature and extent of active patient and public involvement will vary depending on the context of each study/project, however you should include as much detail as possible in your application.
Your application will be assessed by members of a Patient Advisory Group (PAG), who all have personal experience of a heart or circulatory condition(s), either as a patient, carer or family member. The purpose of the PAG is to ensure that people affected by heart and circulatory conditions are able to inform the funding decisions made by both the Clinical Studies Committee (CSC) and Healthcare Implementation Fund (HIF) Committee.
Up to 4 members of the PAG will be assigned to review and score your application. PAG members represent a broad range of heart and circulatory conditions, and where possible, each member will review applications that relate to their own condition(s). PAG members will specifically provide feedback on (including but not limited to):
- The quality of Plain Language summaries (see Tips for writing your Plain Language Summary)
- The importance of the research question/implementation project to people affected by heart and circulatory conditions
- The commitment to/quality of patient and public involvement (PPI), both in the development of the application and proposed plans for ongoing involvement
- Study/project recruitment plans and any barriers to people with heart and circulatory conditions taking part in the research/project (including plans to reach a diverse and representative group of study/project participants)
- PPI budget e.g., whether the applicant has sufficiently budgeted for costs relating to PPI e.g., out-of-pocket expenses and/or payment for involvement (see Budget for PPI)
Each PAG also includes two representatives who are appointed as full members of the CSC/HIF Committees. These members attend and represent the collective views of the PAG at CSC/HIF meetings. As part of discussions to determine the final funding decision, PAG representatives will also comment on whether applicants have sufficiently responded to PAGs comments in the rebuttal.
Tips for writing your Plain Language Summary
The Plain Language Summary (or Plain English Summary) is a part of the application form when applying for a BHF Clinical Study Grant or to the Healthcare Implementation Fund. This is a clear, brief overview of your project and should be written in clear and simple terms. Please ensure that it contains enough detail for Patient Advisory Group (PAG) reviewers to make an informed decision about the study/project.
When considering the accessibility of the Plain Language Summary to a ‘lay audience’, you may wish to consider that in the United Kingdom (UK):
- More than 4 in 10 adults struggle to understand health content written for the public
- 7.1 million adults read at, or below, the level of an average 9-year-old
- The majority of adults are in the 11–14-year old reading age group
When writing your summary:
- Avoid using jargon, abbreviations and technical terms wherever possible – if you have to use them, provide a clear explanation on the first mention.
- Avoid complicated English or uncommon words
- Use active not passive phrases, for example say ‘we will do it’ rather than ‘it will be done by us’
- Keep sentences short - try not to use more than 15 to 20 words per sentence
- Consider breaking up the text e.g., by using bullet points
- Ask someone without a scientific background/subject knowledge to read your draft and advise if anything is unclear
- Consider using online readability tools based on the Flesch-Kincaid readability scale (determines how easy a passage in English is to read)
Refer to NIHR guidance on what to include in your Plain Language Summary.
How can BHF help?
- Connect you with people affected by heart and circulatory conditions and their risk factors. If you would like to share a PPI opportunity with our Heart Voices network, please complete this form. Please note that relevant opportunities are shared with the Heart Voices network on a monthly basis, therefore please give us as much notice as possible to ensure your project can be shared.
- Provide ad-hoc PPI advice. If you would like feedback on your involvement plans or have any questions about PPI, you can get in touch with the PPI Team via [email protected]
Please note: we do not provide support for recruitment of clinical trial/study participants, please refer to NIHR Be Part of Research for this service.
External resources
NIHR briefing notes on public involvement in NHS, health and social care >
A comprehensive guide for researchers new to PPI, as well as those looking to refresh knowledge and skills. This guidance will help you plan, resource and support PPI in research.
NIHR payment guidance for researchers and professionals >
Provides specific guidance for researchers and professionals on Payment for PPI.
UK standards for public involvement >
A set of standards designed to improve the quality and consistency of public involvement in research. They provide a description of the hallmarks of ‘good’ PPI.
NIHR People in Research >
An advertising route for researchers who want to find patients and/or members of the public to get involved in their research. Please note this is different from NIHR Be Part of Research.
NIHR Be Part of Research >
For researchers who want to find potential study/clinical trial participants.
NIHR Plain English Summaries >
Guidance for anyone writing a plain English summary of research.

