Stroke - LACI-2 trial
Could two existing drugs help prevent dementia after lacunar stroke?
The clinical question
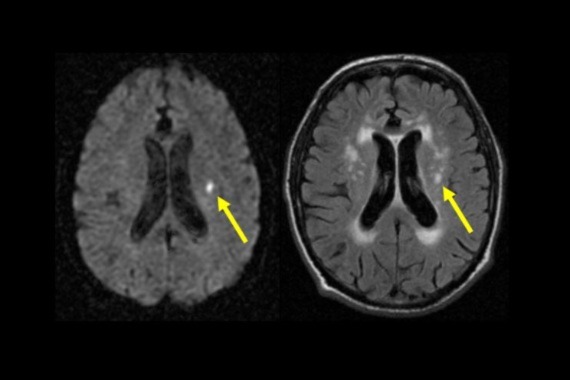
A stroke happens when there is an interruption in blood supply to part of the brain. Lacunar stroke is a type of stroke thought to be caused by cerebral small vessel disease (cSVD), where small blood vessels deep within the brain become damaged. It accounts for about a fifth of all strokes, with at least 35,000 lacunar strokes happening each year in the UK. They can lead to people developing problems with their thinking, memory, movement, and ultimately dementia - but there are currently no proven effective preventive treatments. Overall, cSVD is thought to contribute to around 40 per cent of dementia cases.
Every blood vessel in our body is lined by cells called endothelial cells, which help to keep our circulatory system working properly and protected from damage. However, if endothelial cells become damaged this protective function can be lost – this ‘endothelial dysfunction’ is thought to play a key role in the development of cSVD. Many existing medications used to treat cardiovascular diseases are known to have effects on endothelial cells. For example, isosorbide mononitrate (a nitrate drug used to treat angina) and cilostazol (a weak antiplatelet drug that can be used to treat symptoms of peripheral arterial disease) are thought to help improve endothelial function - so could potentially also help reduce cSVD, and the damage caused by lacunar stroke.
Led by Professor Joanna Wardlaw at the University of Edinburgh, the BHF-funded LACunar Intervention Trial-2 (LACI-2) aimed to explore whether isosorbide mononitrate and cilostazol could be used as treatments for lacunar stroke.
What did the study involve
LACI-2 aimed to enrol 400 people who had experienced a lacunar stroke from 26 UK hospitals. Participants were randomly assigned to take one of the following for up to year (while continuing their normal prescribed medication, such as blood pressure or cholesterol-lowering drugs).
-
Daily isosorbide mononitrate tablets (40-60mg)
-
Daily cilostazol tablets (200mg)
-
Both of the above
-
Neither of the above
Participants were followed up by the trial team by phone and post for up to a year. The main goal of LACI-2 was to assess whether people who had experienced a lacunar stroke would be willing to be enrolled in the trial, and whether 95% of them would be willing to keep taking the medication and remain part of the trial for a year - important information for planning a future, larger clinical trial that could definitively test whether these medications could benefit people who experience a lacunar stroke.
The follow up also involved assessing whether taking isosorbide mononitrate and cilostazol was tolerable and safe for people taking part, and whether there were any differences in ‘clinical outcomes’ between the groups. This included whether they experienced another stroke, the level of difficulty they were experiencing with their brain function (such as their thinking or memory), and quality of life. Participants also had an MRI scan of the brain 12 months after starting the treatment to look at what effects the drugs might have on the small blood vessels within the brain.
What did the study show?
- 363 out of the target of 400 participants were recruited into the trial. Of these, 358 (98.5%) completed one year of follow up, meaning that the trial met its feasibility target.
- Both cilostazol and isosorbide mononitrate appear to be safe and were well tolerated by people with lacunar stroke.
- While this study was not large enough to draw conclusions about the potential benefits of the treatments, the trial did find some evidence of beneficial effects of taking isosorbide mononitrate or cilostazol. For example, people taking isosorbide mononitrate were less likely to have experienced another stroke; and people taking cilostazol tended to be more independent and less likely to be experiencing low mood.
- There was also some evidence of a possible ‘synergistic’ effect between the two drugs, where the beneficial effects appeared to be strengthened if they were taken together. Overall, participants that took both drugs were nearly 20% less likely to have problems with their thinking and memory compared to the group that did not take either drug
Why is the study important?
LACI-2 provided vital evidence that carrying out a larger scale trial to definitively test if isosorbide mononitrate or cilostazol can help people with lacunar stroke would be safe and feasible. The findings also suggest that these medications might have beneficial effects in this patient group - particularly when they’re used in combination - providing hope that they could become some of the first proven treatments for this debilitating condition. The team is now planning to test these drugs in a larger four-year clinical trial, LACI-3.
Professor Wardlaw, who led the trial, said: “Up until now, lacunar strokes have been treated just like other types of stroke, but lacunar stroke is clearly different. Now we understand more about what is triggering these strokes to attack the brain, we’ve been able to focus on treatments that could put halt to this damage.”
“We need to confirm the results of LACI-2 in larger trials before either isosorbide mononitrate or cilostazol can be recommended as a routine treatment. However, as these drugs are already widely available for other circulatory disorders, and inexpensive, it shouldn’t take too long to move our findings from research into everyday clinical practice.”
We hope that our research will lead to effective ways to treat lacunar strokes and potentially prevent some cases of dementia, providing a lifeline for the thousands of people living with these debilitating diseases.Professor Joanna Wardlaw, Chief Investigator, LACI-2
The participant voice
Ian Reynolds, from Edinburgh was one of the 363 people who took part in LACI-2. He had a lacunar stroke in July 2020. He said: “It started with a tingle in my left hand, but within a couple of hours a numbness had spread up my left side and then right up to my face. I had never experienced anything like it before. To be honest, I didn’t even register what was going on. When the doctors told me I might have had a stroke, I thought ‘Me? No way’.”
Once scans confirmed he had had a lacunar stroke, Ian was asked to take part in the trial. He said yes immediately. “The opportunity to help people who have a lacunar stroke in the future by taking part was really important to me. I also learned a lot about what I had been through, and the researchers and nurses were always happy to explain anything about my stroke or the trial.”
Ian was part of the group that took cilostazol alone. Two and a half years after his stroke, he still has numbness and weakness in his left arm and leg. However, he was able to return to work as a driver six months after his stroke. “I’m determined that this will not stop me living my life” he says. “My experience could have been very different, and I realise just how lucky I have been. It looks like the drugs have helped a lot of people, which can only be good news.”
Far too many are living with the after-effects of this type of stroke, so finding a treatment would be fantastic.Ian Reynolds, Participant in LACI-2
Study details
“LACunar Intervention (LACI) trial: assessment of safety and efficacy of cilostazol and isosorbide mononitrate to prevent recurrent lacunar stroke and progression of cerebral small vessel disease”
Award reference: CS/15/5/31475
Principal investigator: Professor Joanna Wardlaw
Trial registration number: NCT03451591
Publication details
Isosorbide Mononitrate and Cilostazol Treatment in Patients With Symptomatic Cerebral Small Vessel Disease: The Lacunar Intervention Trial-2 (LACI-2) Randomized Clinical Trial. JAMA Neurol. 2023 Jul 1;80(7):682-692.

