

Scientists funded by us share stunning images of their cutting-edge research into finding a desperately needed cure for heart failure, a devastating condition affecting nearly one million people in the UK. Each image reflects work tackling heart failure with different approaches to regenerative medicine that uses ways to teach the heart to repair itself.
We have been announced as charity of the year for the 2022 TCS London Marathon. The £3 million we hopes to raise will fund eight projects, including these six, all aimed at finding ways to cure heart failure.
Heart failure happens when part of the heart is damaged, and it becomes more difficult to effectively pump blood around the body. This is commonly caused by a heart attack, high blood pressure or an inherited condition. It is a debilitating condition that makes everyday tasks very difficult.
Stimulating heart regeneration by Professor Mauro Giacca, King’s College London
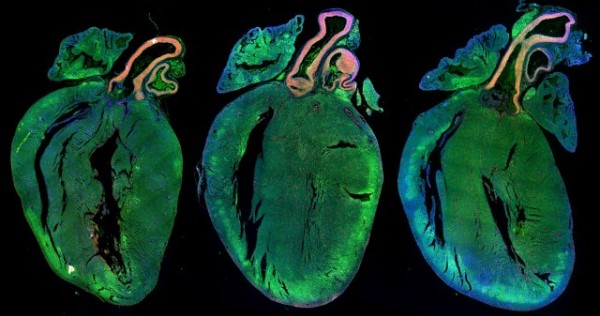
These glowing hearts show the effects of stimulating mouse hearts to regenerate. The two hearts on the right have been injected with microRNAs (small molecules that turn genes off), which cause heart muscle cells to multiply. This stimulation thickens the heart muscle, making it stronger. The heart on the left-hand side looks smaller as it has not been given these microRNAs.

These luminous green blobs with brightly coloured spots may look like an alien life form from outer space but they are heart muscle cells. They have also been stimulated by the same microRNAs as the mouse hearts. The red dots show which cells are multiplying to ultimately make the heart muscle stronger.
Professor Giacca and his team hope that injecting microRNAs into the heart will stimulate heart cells to regenerate and repair the damage seen in people with heart failure.
Developing blood vessels in zebrafish by Dr Sarah De Val, University of Oxford

This may look like a sea monster from the deep, but it is the developing blood vessels of a two-day old zebrafish embryo. All of the blood and lymphatic vessels are labelled with a red fluorescent protein. The veins are also labelled with a green fluorescent protein, making the veins glow yellow and the arteries glow red.
Dr De Val and her team study the zebrafish in this way to better understand how blood vessels develop, with the aim of manipulating blood vessel growth in the human heart so that it can recover better after a heart attack.
Migrating clone cells by Dr Mairi Brittan, University of Edinburgh

These colourful streamers show cells on the inside of the blood vessels (endothelial cells) in the heart that have copied and are moving to new locations. These copies, known as ‘clone cells’, move to areas that don’t have enough oxygen. There, they create new blood and lymphatic vessels.
Dr Brittan and her team hope that by finding ways to stimulate these clone cells after a heart attack, the heart can learn to rewire its blood vessels to provide damaged areas of the heart with more oxygen. This increase in oxygen and nutrients could save heart muscle and prevent heart failure.
Beating heart patches in a dish by Professor Sanjay Sinha, University of Cambridge.

These undulating waves of heart cells speckled with white nuclei are part of a beating heart patch grown in a dish in Professor Sinha’s laboratory. He and his team are using stem cells to grow patches of real heart tissue. They hope to apply them onto damaged areas of the heart so the heart can repair itself.
Branching blood vessels in the heart by Dr Joaquim Vieira, University of Oxford

This beautiful image that resembles tree roots branching out shows the development of the blood vessels on the outside surface of the heart called the epicardium.
Dr Vieira hopes to understand processes in the embryo which cause the heart to repair itself after damage. He will be investigating how cells from the epicardium are ‘switched on’ in a process called the epithelial-to-mesenchymal transition (EMT). By understanding the EMT process in the heart, Dr Vieira believes it might be possible to switch the genes on again, helping to heal damaged hearts.
Heart muscle grown from stem cells by Professor Stefan Hoppler, University of Aberdeen

Here we can see heart muscle cells that have been grown from stem cells. The red strands show a protein called Troponin T, which is crucial for the heart muscle to contract and relax.
Professor Hoppler and his team are using these cells to mimic how heart muscle develops in the womb. Heart muscle cells grown like this in a dish could one day help patients who have had a heart attack and improve their recovery.
Professor Metin Avkiran, our Associate Medical Director, said:
“Heart failure is a debilitating condition that dramatically affects the lives of almost 1 million people in the UK. BHF-funded research has spear-headed treatments to give people with heart failure longer, healthier lives, but there is no cure. Regenerative medicine offers that hope.
“The money raised by the 2022 TCS London Marathon will enable these researchers to push the boundaries of medicine by finding ways to teach the heart to repair itself. Unlocking these secrets could help heal hearts and transform the outcomes for people living with devastating heart failure.”


