The science behind the cure
CureHeart brings together world-leading experts and cutting-edge technologies to deliver a revolution in precision cardiology. Explore the team’s bold aims, and the science powering their progress.
What's on this page:
- How do mistakes in DNA cause inherited heart muscle diseases?
- Gene-editing technologies to correct mutations in the DNA
- The packaging system that can carry gene-editing tools into heart muscle cells
- Choosing the best method to deliver the package to the beating heart
- Covering all bases to ensure success
- The reality of a cure
In some cases, the symptoms and risks of inherited heart muscle diseases can be managed.
To deliver a cure, we must go deeper, right to the heart of the problem. To mistakes in the DNA itself.
How do mistakes in DNA cause inherited heart muscle diseases?
All cells of the body contain DNA – the genetic instruction manual that tells our bodies how to build proteins. These are molecules that perform important functions in all our cells, which in turn form organs, including the heart.
The instruction manual is formed of an alphabet made up of just four letters, or bases, labelled A, C, G, and T. Chains of bases pair up – A with T, G with C - to form long strands of DNA in its iconic double helix structure. Three base letters form one word, which are strung together to form the sentences that build complex proteins.
There are 3 billion base pairs in human DNA. These can become damaged, and mistakes can occur when DNA is copied as the cell divides. Such mistakes are called mutations.
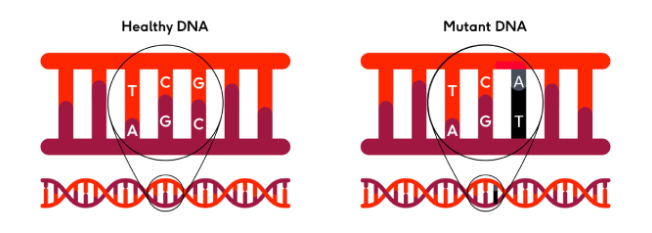
Mutations are like spelling mistakes. There might be an A instead of a G, changing the meaning of the words in the instruction manual.
Sometimes, 1 or more letters might be missed out and so the words may no longer make sense because the bases are read in threes. If the words no longer make sense, then the instructions may be read incorrectly, producing faulty proteins or not producing them at all.
This can lead to disease.

In some cases, mutations can be passed down through families and lead to inherited diseases like genetic cardiomyopathies. We inherit one copy of our genes from each parent, and usually only one copy of the gene has a mistake in it. For some genes we need both copies to work for enough protein to be made and a mistake in one of them may reduce production.
Sometimes a faulty protein is made by the mutated gene, and this stops the normal proteins from the healthy copy doing their job too. Whatever the reason, these mistakes in the DNA mean the heart muscle cells don’t work as they should.
Correcting the spelling mistakes in heart muscle cells could allow them to work properly again, but this would require a tool that gets inside the correct cell and only corrects the mutated DNA.
The CureHeart team are using cutting-edge technology to tackle the problem head on.
Gene-editing technologies to correct mutations in the DNA
Incredibly, a tool to edit DNA already exists. It was developed from a defence system that bacteria use to chop up the DNA of invading viruses and so protect themselves from harm.
Scientists have been adapting these natural ‘genetic scissors,’ called CRISPR/Cas9, to make more precise changes to DNA.
Instead of chopping it up, these improvements have allowed scientists to alter DNA, letter by letter. Base editing acts like a molecular eraser, changing one typo in the code. Prime editing acts more like a word processor, finding and replacing whole sections of DNA..jpg?h=auto&w=100%25&rev=74b0c5f36d2441ad94b795e437a27a08&hash=674787DA2248B38A08A49BA71C9ABEB5)
CureHeart is exploring these technologies to correct the mutations that cause inherited heart muscle diseases.
The packaging system that can carry gene-editing tools into heart muscle cells
Gene editing machinery isn’t the only scientific technology that has been inspired by the natural world.
Scientists have long been able to engineer viruses to use their shells for delivering new sequences of DNA directly into cells. In this case, the instructions to build editors need to be carried into heart muscle cells.
However, there are challenges in getting them to the right place. Some engineered viruses can reach the heart but are quickly removed by our body. Others can last much longer, but the immune system eventually attacks and destroys them.
Current technologies are also limited by the amount of cargo they can carry, which is a particular challenge when trying to package the bulky instructions for gene-editing tools.
The CureHeart team will be exploring the best way to engineer these clever delivery systems, looking for the safest and most efficient method to deliver gene editing tools into heart muscle cells.
Choosing the best method to deliver the package to the beating heart
CureHeart’s goal is to edit the genetic mutations in heart muscle cells that cause damage and disease. But getting the editing machinery into these cells presents another significant hurdle.
A simple injection into the bloodstream could be the answer, as blood travels through the heart as part of its journey around the body. But the treatment could be filtered out by the liver before it can enter enough heart muscle cells. Another concern is that it could be taken up by other organs of the body where it’s not needed.
Direct delivery to the heart may seem like a better option, but this is a much more complicated and expensive procedure that also carries its own risks.
The CureHeart team will test different methods of delivery, to determine the safest, easiest, and most effective treatment approach.
Covering all bases to ensure success
One of the strengths of the CureHeart project is the number of options it’s exploring.
While the gene-editing approach could permanently correct mistakes in DNA, there’s another option which may require repeat treatments rather than a single dose, but could avoid the need for more complicated delivery methods.
Some spelling mistakes can produce faulty proteins that block healthy ones from doing their job properly. These mistakes are called dominant negative mutations.
The CureHeart team is testing a technique called antisense silencing that doesn’t change the DNA code, but stops the faulty protein being built. This would protect the function of the healthy protein, allowing the heart muscle cell to return to a healthier state.
With repeated treatment, this could represent a new therapy to suppress the effects of the dominant negative mutations affecting a significant proportion of people living with these diseases. Similar approaches are already being used to treat other diseases, signalling hope for those living with inherited heart muscle diseases.
The reality of a cure
The CureHeart team have bold aims, but they are confident that they can achieve them.
They are bringing together experts across disciplines and continents to bring a cure for inherited heart muscle diseases to early clinical trials in the next 5 years.
Meet the team behind CureHeart
Find out more about the CureHeart project on the team's website

