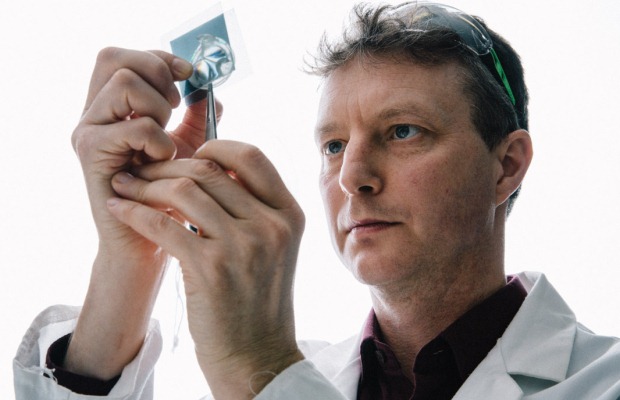
What’s the perfect material for a heart valve? It needs to be strong yet flexible, capable of opening and closing 200 million times or more, compatible with the human body and able to let blood flow easily through it.
Dr Geoff Moggridge thinks he’s found the solution. On his desk in the Cambridge University chemical engineering department is a colourless, semi-transparent sheet. It’s a block copolymer – two kinds of polymer (a large molecule made of many repeated units – nylon, cellulose, wool and DNA are all examples), chemically bonded together.
Dr Moggridge’s copolymer consists of a glassy material, which self-assembles into fibres, bonded to a rubbery material. “It is a bit like fibreglass, but on a very small scale and well controlled,” he says.
The material was invented in the 1970s but only became easy to manufacture recently. Tug it one way, it stretches like a sturdy elastic band: another way, you get only firm resistance. “It is quite striking to have a material that is so different in one direction to another,” says Dr Moggridge. “It has to be strong and flexible. If you make it too strong it won’t open and close properly, which is one of the problems that means people need heart valve replacements in the first place. You couldn’t make a heart valve out of steel, for example.”
The new valve is formed by injection moulding. Material is melted and inserted into a mould, where it cools and sets. “If you can get the fibres in the material lined up so that the direction of greatest strength is in line with the greatest stress on the valve, that would be really useful,” says Dr Moggridge. “That is what is different about what we are doing. A natural valve has that kind of property; we are imitating what a natural material does.”
The end of anticoagulation?
A key advantage of the copolymer heart valve over mechanical valves is that patients won’t need lifelong anticoagulant medications. Anticoagulants reduce clotting but carry a risk of bleeding and, in the case of warfarin, require regular monitoring and blood tests. Dr Moggridge is “reasonably confident” patients with his valve won’t need the drugs long-term.
Clotting is a risk in existing mechanical valves, which are usually made from carbon or titanium ‘leaflets’ (flaps) attached to a Teflon or polyester ring. “The blood swirls around mechanical valves, because of the way they open,” says Dr Moggridge. “We think it’s this rotation in the blood flow that leads to clotting.”
The Cambridge team has done experiments to mimic blood flow through their valve. It closely resembles the shape of a natural valve, so blood flow is natural too, reducing clotting risk. A microscopic coating of heparin (an anticoagulant) on the valve reduces this risk even further.
It could also last longer than animal-tissue valves, reducing need for repeat surgery, although more testing is needed. Once the valve is perfected, it should be easier to manufacture and the end result more consistent than animal valves.

Different approach
Dr Moggridge and his team are working on a second valve, to be used in transcatheter valve surgery. This is where a new valve is delivered through a catheter (a hollow tube) inserted directly into the heart or through a blood vessel in the leg, and positioned on top of the old one.
The project, funded by a further BHF grant worth £248,000, is to make a removable transcatheter valve. Currently, if a transcatheter valve wears out, it can only be removed by open heart surgery. The first removable valve will use animal-tissue leaflets, but future versions could use the block copolymer. “There is some evidence that when you squash a biological valve to deliver it with a catheter it can get damaged,” says Dr Moggridge.
Dr Moggridge is a chemical engineer, not a heart surgeon, so facts like this are relatively new to him. In fact, he was initially turned down for a BHF grant. “They said: ‘Go away and find a medical collaborator to work with you’,” he explains. “So I worked with Suku Nair, who at the time was a surgeon at Papworth Hospital and is now based at Newcastle.”
During ‘traditional’ valve replacement, the surgeon sews the new valve into place. “It was so useful to watch the details of what a surgeon does during the operation and what they were concerned about,” says Dr Moggridge. “Like, how is he going to sew it in, will the needle go through the material, and will it tear?”
Rigorous testing
Both new valves are going through rigorous testing. Replacement heart valves are subject to International Organisation for Standardisation (ISO) standards, just like A4 paper and agricultural machinery. Currently, the ISO standard says: “There is, as yet, no heart valve substitute which can be regarded as ideal.”
It stipulates that a replacement valve must last 200 million heartbeats – about five years. Dr Moggridge is aiming for 400 million, or 10 years, as a minimum.
How can you test a heart valve outside the human body? You can use a homemade pulse duplicator (where water and glycerol flow through the valve to imitate blood) and then a durability tester. Dr Moggridge’s team have a new durability tester, costing £50,000 and funded by a BHF grant. “The tester can go at 30 times normal speed and test six valves at the same time,” he says. “It means we can simulate five years of use in six months.”
If testing is successful, trials in pigs or sheep will start in Bristol next year. Dr Moggridge says it could be “a matter of years rather than decades” until the valve is available for patients.
Aside from his lab work, Dr Moggridge teaches in King’s College, in sight of the world-famous chapel and the 18th-century Gibbs building, where he used to live. The surroundings are a reminder of regal wealth, but funding no longer comes from royalty.
Dr Moggridge is grateful to the BHF for its support. “The current grant is paying for two post-doctoral scientists, Joanna Stasiak and Marta Serrani, who do most of the work. It will also pay for the animal trials,” he says.
“The work wouldn’t have happened at all without BHF funding.”













